Learning Objectives
In each iteration, the masses of each element will be added and stored in the mass object # If not, just add that element's mass to the sum. Else: masa += elementmass return masa No problem is the molecule looks like 'C14-H10-O2' BUT if some of the elements have not one digit after the element, Python reply with this error. Calculating Atomic Mass. Protons and Neutrons make up the mass of an atom.% Progress. This indicates how strong in your memory this concept is. 9/20/2020 Calculating Average Atomic Mass CK-12 Foundation 3/6 nearly all oxygen-16. Nearly all oxygen-16. For many other elements many other elements, however, more than one isotope may exist in more substantial quantities. Chlorine Chlorine (a t omic number number 17) is a yellowish-green toxic gas.About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass.
- Define atomic mass.
- Calculate atomic mass given relevant information about the isotopes.
Have you ever tried to move a boulder?
You have a pile of rocks to move and need to decide what equipment you want to rent to move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop. The amount of each kind of rock will also determine how much time you will need to get the job done. Knowing the relative amounts of large, medium, and small rocks can be very useful in deciding how to approach the job.
Most elements occur naturally as a mixture of two or more isotopes. Table below shows the natural isotopes of several elements, along with the percent natural abundance of each.
| Element | Isotope (symbol) | Percent natural abundance | Atomic mass (amu) | Average atomic mass (amu) |
| Hydrogen | [latex]^1_1text{H}[/latex] | 99.985 | 1.0078 | 1.0079 |
| [latex]^2_1text{H}[/latex] | 0.015 | 2.0141 | ||
| [latex]^3_1text{H}[/latex] | negligible | 3.0160 | ||
| Carbon | [latex]^{12}_6text{C}[/latex] | 98.89 | 12.000 | 12.011 |
| [latex]^{13}_6text{C}[/latex] | 1.11 | 13.003 | ||
| [latex]^{14}_6text{C}[/latex] | trace | 14.003 | ||
| Oxygen | [latex]^{16}_8text{O}[/latex] | 99.759 | 15.995 | 15.999 |
| [latex]^{17}_8text{O}[/latex] | 0.037 | 16.995 | ||
| [latex]^{18}_8text{O}[/latex] | 0.204 | 17.999 | ||
| Chlorine | [latex]^{35}_{17}text{Cl}[/latex] | 75.77 | 34.969 | 35.453 |
| [latex]^{37}_{17}text{Cl}[/latex] | 24.23 | 36.966 | ||
| Copper | [latex]^{63}_{29}text{Cu}[/latex] | 69.17 | 62.930 | 63.546 |
| [latex]^{65}_{29}text{Cu}[/latex] | 30.83 | 64.928 |
For some elements, one particular isotope predominates greatly over the other isotopes. Naturally occurring hydrogen is nearly all hydrogen-1 and naturally occurring oxygen is nearly all oxygen-16. For many other elements, however, more than one isotope may exist in more substantial quantities. Chlorine (atomic number 17) is a yellowish-green toxic gas. About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a mass number of 37. Were you to simply calculate the arithmetic average of the precise atomic masses, you would get 36.
[latex]displaystylefrac{left(34.969+36.966right)}{2}=35.968text{ amu}[/latex]
Clearly the actual average atomic mass from the last column of the table is significantly lower. Why? We need to take into account the percent natural abundances of each isotope in order to calculate what is called the weighted average. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. The sample problem below demonstrates how to calculate the atomic mass of chlorine.
Sample Problem: Calculating Atomic Mass
Use the atomic masses of each of the two isotopes of chlorine along with their percent abundances to calculate the average atomic mass of chlorine.
Step 1: List the known and unknown quantities and plan the problem.
Known
- chlorine-35: atomic mass = 34.969 amu and % abundance = 75.77%
- chlorine-37: atomic mass = 36.966 amu and % abundance = 24.23%
Unknown
- Average atomic mass of chlorine

Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
Step 2: Calculate
| chlorine-35 | 0.7577 × 34.969 = 26.50 amu |
| chlorine-37 | 0.2423 × 26.966 = 8.957 amu |
| average atomic mass | 26.50 + 8.957 = 35.45 amu |
Note: Applying significant figure rules results in the 35.45 amu result without excessive rounding error. In one step:
(0.7577 × 34.969) + (0.2423 × 36.966) = 35.45 amu
Step 3: Think about your result.
The calculated average atomic mass is closer to 35 than to 37 because a greater percentage of naturally occurring chlorine atoms have the mass number of 35. It agrees with the value from the Table above .
Watch these videos to learn more about these calculations:
Calculating Atomic Mass Pdf
Summary
Calculating Atomic Mass Problems
- The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
- Calculations of atomic mass use the percent abundance of each isotope.
Practice
Click on the link below to get some experience in atomic mass determinations:
Review
- Define atomic mass.
- What information do you need to calculate atomic mass for an element?
- Calculate the atomic mass for carbon using the data provided in Table below.
mass number | exact weight | percent abundance |
12 | 12.000000 | 98.90 |
13 | 13.003355 | 1.10 |
Glossary
- atomic mass: The weighted average of the atomic masses of the naturally occurring isotopes of that element.
- percent abundance: To calculate the weighted average, take into account the percent natural abundances of each isotope. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
References
- Devon Fyson, based on image created by the U.S. Geological Survey. http://commons.wikimedia.org/wiki/File:Mass_Spectrometer_Schematic.svg.
- George M. Groutas. http://www.flickr.com/photos/jorge-11/4777791888/.
How do you calculate atomic mass?
To calculate the atomic mass of an element, we have to calculate how much each isotope contributes to the mass of the atom. To accomplish this, we usually use an approach called the weighted average. The weighted average takes into account the mass and percentage abundance of each isotope.
What’s percentage abundance?
It is the proportion of atoms of an isotope in a sample of an element taken from the natural world. Percentage abundance is always reported as a percentage, and it is calculated as: (number of atoms of an isotope) divided by (the total number of atoms of all isotopes of that element) multiplied by 100. Percentage abundance usually can be divided by 100 to get fractional abundance.
How do you use weighted average to calculate atomic mass?
To use weighted average, we must take into account the mass and percentage abundance of each isotope. Let’s use the data in the following table to show how weighted average is used to calculate the atomic mass of oxygen.
Solution
To calculate the atomic mass of oxygen using the data in the above table, we must first
- multiply the mass of each isotope by its corresponding natural abundance (percentage abundance). But, since the abundance is in %, you must also divide each abundance value by 100.
And second,
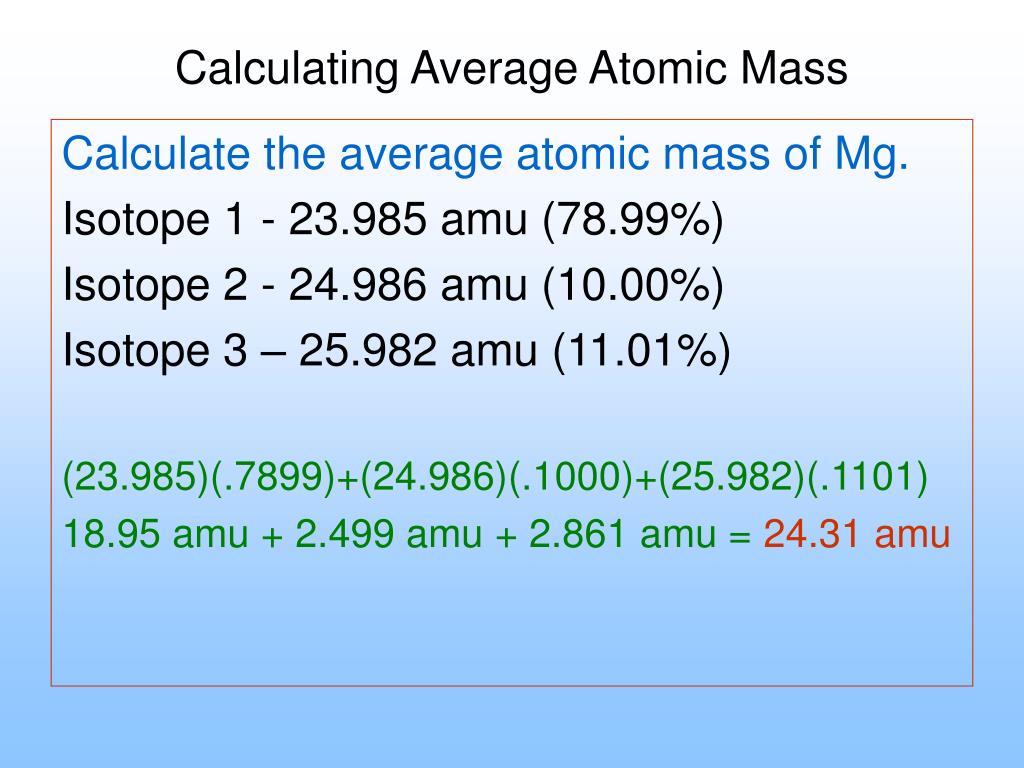
- Sum the result to get the atomic mass of the element
Thus,
Atomic mass of oxygen = 15.995 amu (99.76/100) + 16.999 amu (.04/100) + 17.999 amu (.2/100)
= 15.956612 amu + 0.0067996 amu + 0.035998 amu
= 15.9994096 amu
= 16.00 amu
Note that the abundance in percent always add up to 100 %. Meaning if the previous question had left out the percentage abundance value for oxygen-18, you could have gotten it by subtracting the sum of the percentage abundance for oxygen-16 and oxygen-17 from 100% to get the percentage abundance for oxygen-18.
That is: 100% – (99.76% + .04%) = 0.2% for oxygen-18
Generally, you can apply this approach to figure out missing percentage abundance when you know the percentage abundance values for all, but one isotope.


If you examine the atomic masses on the periodic table, you will notice that they are fractional. Why are they fractional? They are fractional because atoms exist as isotopes. And isotopes do not have the same mass and abundance in nature. As a result, to calculate the atomic mass of an element, we have to calculate how much each isotope contributes to the mass of the atom.
Why are there no units of grams attached to the atomic mass of oxygen?
There are no units attached to atomic masses because atomic masses are relative atomic masses. Relative in this sense means one thing is compared to another. So, relative atomic mass means the mass of one atom is compared to the mass of another atom.
The atom to which other atoms are compared to is usually called the standard. At present, an isotope of carbon called carbon-12 (C-12) is selected as the standard and assigned an atomic mass of exactly 12 amu, where amu stands for atomic mass units. Therefore, the mass of every other atom on the periodic table is determined by how light or heavy it is when compared to the mass of C-12.
What instrument is used to measure the relative masses of atoms?
At present, mass spectrometry is the technique used to measure the relative masses of atoms and their percentage abundance in nature. In a mass spectrometer, atoms interact with a magnetic field and separate according to their mass to charge ratio. As they separate according to this ratio, their percentage abundance and relative atomic masses can be calculated. Let’s use the following example to illustrate how the relative mass of an atom is calculated using carbon-12 as the standard.
Example
If a chemist measured a sample in a mass spectrometer and determined that the mass ratio of 28Si (silicon-28) to 12C (carbon-12) is: 2.33, calculate the mass of 28Si?
Solution
Since the mass of carbon-12 is is assigned a value of 12, then it follows that the mass of 28Si = mass ratio of (28Si/12C) multiplied by mass of 12C
Thus, mass of 28Si = 2.33 x 12 amu = 27.98 amu
To learn more about atomic mass and Avogadro’s number, click here.
